


"If a large number of schools, hospitals, office buildings and residences were modified [with UVGI]…A number of airborne respiratory diseases could be eradicated…by interrupting the transmission cycle. Reducing the transmission rate sufficiently would…Halt epidemics in their path."
- Dr. Władysław Jan Kowalski, Pennsylvania State University's Indoor Environment Center
Damp, dark HVAC systems are the perfect breeding ground for mold and bacteria. Each time the HVAC system runs, these contaminants and airborne viruses are circulated throughout the entire home. Research has shown that home occupants exposed to such contaminants can develop health issues, including sinus congestion and headaches, allergies and asthma, upper respiratory ailments, along with colds and flu. According to the World Health Organization, ailments caused by these contaminants account for a substantial portion of absences from school and work, and can lead to lower productivity. ALTRU-V® products from UV Solutions, continuously destroy contaminants in the HVAC systems, protecting building occupants and technicians.
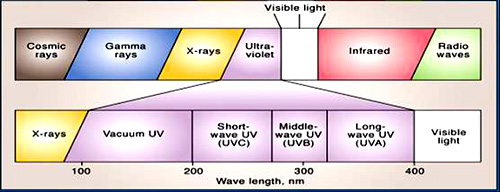
UV energy is found in the electromagnetic spectrum between visible light and x-rays and is sometimes described as invisible radiation. Germicidal protection is delivered at 254 nanometers in the light spectrum and is known as UV-C. Its photos have just the right wavelength to physically destroy the DNA of microorganisms. It is a safe, tested technology that uses lamps, similar to fluorescent tubes, to generate UV-C light to kill bacteria, viruses and mold spores. UV light penetrates the outer cell membrane of the microorganism, passes through the cell body, reaches the DNA and alters the genetic material. Without chemicals the microorganism is destroyed and is unable to reproduce. UV-C, combined with the right filter technology, is the optimal solution for cost effective and safe maintenance for HVAC systems.
ALTRU-V® products from UV Solutions are guaranteed to optimize HVAC performance resulting in overall lower operating costs, safety, reliability, and cleaner air.
Cooling (Evaporative) Coils are a critical part of any HVAC system. They also act as a "Mechanical Petri Dish" for the growth of organic contaminates and allergens like fungi, molds and bacteria that reduce the operating efficiency of the system. This growth is a natural occurring element of any HVAC system. Traditional coil cleaning is costly, only effective for a short term, labor intensive, requires the use of chemicals and eventually leads to system performance degradation. Combining the calculated positioning of the UV-C lamps for 100% coverage of the surface area with the proper specification of intensity and it is relatively easy to deliver UV-C on a surface area that over time will destroy any microorganism residing there.
Traditional coil cleaning is then no longer needed, HVAC system performance is restored and harmful chemicals are not used in the air stream. Additionally, the use of normal or low intensity UV-C lamps becomes a very cost effective means for using the application and accomplishing the goals of the end user.
Ultraviolet (UV) light is electromagnetic radiation with a wavelength shorter than that of visible light, but longer than soft X-rays. It can be subdivided into near UV (400–200 nm wavelength; abbrev. NUV), far or vacuum UV (200–10 nm; abbrev. FUV or VUV), and extreme UV (1–31 nm; abbrev. EUV or XUV).
The name means "beyond violet" (from Latin ultra, "beyond"), violet being the color of the shortest wavelengths of visible light.
The discovery of UV radiation was intimately associated with the observation that silver salts darken when exposed to sunlight. In 1801 the German physicist Johann Wilhelm Ritter made the hallmark observation that invisible rays just beyond the violet end of the visible spectrum were especially effective at darkening silver chloride-soaked paper. He called them "deoxidizing rays" to emphasize their chemical reactivity and to distinguish them from "heat rays" at the other end of the visible spectrum. The simpler term "chemical rays" was adopted shortly thereafter, and it remained popular throughout the 19th century. The terms chemical and heat rays were eventually dropped in favor of ultraviolet and infrared radiation, respectively.
When considering the effect of UV radiation on human health and the environment, the range of UV wavelengths is often subdivided into UVA (400–315 nm), also called Long Wave or "black light"; UVB (315–280 nm), also called Medium Wave; and UV-C (< 280 nm), also called Short Wave or "germicidal". See 1 E-7 m for a list of objects of comparable sizes.In photolithography, in laser technology, etc., the term deep ultraviolet or DUV refers to wavelengths below 300 nm.
Some of the UV wavelengths are colloquially called black light, as it is invisible to the human eye. Some animals, including birds, reptiles, and insects such as bees, can see into the near ultraviolet. Many fruits, flowers, and seeds stand out more strongly from the background in ultraviolet wavelengths as compared to human color vision. Scorpions glow or take on a yellow to green color under UV illumination. Many birds have patterns in their plumage that are invisible at usual wavelengths but observable in ultraviolet, and the urine of some animals is much easier to spot with ultraviolet.

The Sun emits ultraviolet radiation in the UVA, UVB, and UVC bands, but because of absorption in the atmosphere's ozone layer, 99% of the ultraviolet radiation that reaches the Earth's surface is UVA. (Some of the UVC light is responsible for the generation of the ozone.)Ordinary glass is partially transparent to UVA but is opaque to shorter wavelengths while Silica or quartz glass, depending on quality, can be transparent even to vacuum UV wavelengths. Ordinary window glass passes about 90% of the light above 350 nm, but blocks over 90% of the light below 300 nm[1][2][3].
The onset of vacuum UV, 200 nm, is defined by the fact that ordinary air is opaque below this wavelength. This opacity is due to the strong absorption of light of these wavelengths by oxygen in the air. Pure nitrogen (less than about 10 ppm oxygen) is transparent to wavelengths in the range of about 150–200 nm. This has wide practical significance now that semiconductor manufacturing processes are using wavelengths shorter than 200 nm. By working in oxygen-free gas, the equipment does not have to be built to withstand the pressure differences required to work in a vacuum. Some other scientific instruments, such as circular dichroism spectrometers, are also commonly nitrogen purged and operate in this spectral region.
Extreme UV is characterized by a transition in the physics of interaction with matter: wavelengths longer than about 30 nm interact mainly with the chemical valence electrons of matter, while wavelengths shorter than that interact mainly with inner shell electrons and nuclei. The long end of the EUV/XUV spectrum is set by a prominent He+ spectral line at 30.4nm. XUV is strongly absorbed by most known materials, but it is possible to synthesize multilayer optics that reflect up to about 50% of XUV radiation at normal incidence. This technology has been used to make telescopes for solar imaging; it was pioneered by the NIXT and MSSTA sounding rockets in the 1990s; (current examples are SOHO/EIT and TRACE) and for nanolithography (printing of traces and devices on microchips).
A positive effect of UVB light is that it induces the production of vitamin D in the skin. It has been estimated[4] that tens of thousands of premature deaths occur in the US annually from a range of cancers due to insufficient UVB exposure (via vitamin D deficiency). Another effect of vitamin D deficiency is osteomalacia (rickets), which can result in bone pain, difficulty in weight bearing and sometimes fractures.
Ultraviolet radiation has other medical applications, in the treatment of skin conditions such as psoriasis and vitiligo. UVB and UVA radiation can be used, in conjunction with psoralens (PUVA treatment). In cases of psoriasis and vitiligo UV light with wavelength of 311 nm is most effective.
In humans, prolonged exposure to solar UV radiation may result in acute and chronic health effects on the skin, eye, and immune system[5]. UVC rays are the highest energy, most dangerous type of ultraviolet light. Little attention has been given to UVC rays in the past since they are filtered out by the atmosphere. However, their use in equipment such as pond sterilization units may pose an exposure risk, if the lamp is switched on outside of its enclosed pond sterilization unit.
Ultraviolet photons harm the DNA molecules of living organisms in different ways. In one common damage event, adjacent bases bond with each other, instead of across the "ladder". This makes a bulge, and the distorted DNA molecule does not function properly.
A bird appears on every Visa credit card when held under a UV light source. A black light is a lamp that emits long wave UV radiation and very little visible light. Fluorescent black lights are typically made in the same fashion as normal fluorescent lights except that only one phosphor is used and the normally clear glass envelope of the bulb is replaced by a deep bluish purple glass called Wood's glass.
To thwart counterfeiters, sensitive documents (e.g. credit cards, driver's licenses, passports) may also include a UV watermark that can only be seen when viewed under a UV-emitting light. Passports issued by most countries usually contain UV sensitive inks and security threads. Visa stamps and stickers such as those issued by Ukraine contain large and detailed seals invisible to the naked eye under normal lights, but strongly visible under UV illumination. Passports issued by the United States have the UV sensitive threads on the last page of the passport along with the barcode.
Fluorescent lamps produce UV radiation by ionizing low-pressure mercury vapor. A phosphorescent coating on the inside of the tubes absorbs the UV and converts it to visible light.
The main mercury emission wavelength is in the UVC range. Unshielded exposure of the skin or eyes to mercury arc lamps that do not have a conversion phosphor is quite dangerous.
The light from a mercury lamp is predominantly at discrete wavelengths. Other practical UV sources with more continuous emission spectra include xenon arc lamps (commonly used as sunlight simulators), deuterium arc lamps, mercury-xenon arc lamps, metal-halide arc lamps, and tungsten-halogen incandescent lamps.
In astronomy, very hot objects preferentially emit UV radiation (see Wien's law). However, the same ozone layer that protects us causes difficulties for astronomers observing from the Earth, so most UV observations are made from space. (see UV astronomy, space observatory)
Ultraviolet fly traps are used for the elimination of various small flying insects. They are attracted to the UV light and are killed using an electrical shock or trapped once they come into contact with the device.
UV/VIS spectroscopy is widely used as a technique in chemistry, for analysis of chemical structure, most notably conjugated systems. UV radiation is often used in visible spectrophotometry to determine the existence of fluorescence a given sample.
Ultraviolet lamps are also used in analyzing minerals, gems, and in other detective work including authentication of various collectibles. Materials may look the same under visible light, but fluoresce to different degrees under ultraviolet light; or may fluoresce differently under short wave ultraviolet versus long wave ultraviolet. UV fluorescent dyes are used in many applications (for example, biochemistry and forensics). The fluorescent protein Green Fluorescent Protein (GFP) is often used in genetics as a marker. Many substances, proteins for instance, have significant light absorption bands in the ultraviolet that are of use and interest in biochemistry and related fields. UV-capable spectrophotometers are common in such laboratories.
Ultraviolet radiation is used for very fine resolution photolithography, a procedure where a chemical known as a photoresist is exposed to UV radiation which has passed through a mask. The light allows chemical reactions to take place in the photoresist, and after development (a step that either removes the exposed or unexposed photoresist), a geometric pattern which is determined by the mask remains on the sample. Further steps may then be taken to "etch" away parts of the sample with no photoresist remaining.
UV radiation is used extensively in the electronics industry because photolithography is used in the manufacture of semiconductors, integrated circuit components[9] and printed circuit boards.
A new application of UV is to detect corona discharge (often simply called "corona") on electrical apparatus. Degradation of insulation of electrical apparatus or pollution causes corona, wherein a strong electric field ionizes the air and excites nitrogen molecules, causing the emission of ultraviolet radiation. The corona degrades the insulation level of the apparatus. Corona produces ozone and to a lesser extent nitrogen oxide which may subsequently react with water in the air to form nitrous acid and nitric acid vapor in the surrounding air[10].
Ultraviolet Germicidal Irradiation
Ultraviolet lamps are used to sterilize workspaces and tools used in biology laboratories and medical facilities. Commercially-available low pressure mercury-vapor lamps emit about 86% of their light at 254 nanometers (nm) which coincides very well with one of the two peaks of the germicidal effectiveness curve (i.e., effectiveness for UV absorption by DNA). One of these peaks is at about 265 nm and the other is at about 185 nm. Although 185 nm is better absorbed by DNA, the quartz glass used in commercially-available lamps, as well as environmental media such as water, are more opaque to 185 nm than 254 nm (C. von Sonntag et al., 1992). UV light at these germicidal wavelengths causes adjacent thymine molecules on DNA to dimerize, if enough of these defects accumulate on a microorganism's DNA its replication is inhibited, thereby rendering it harmless (even though the organism may not be killed outright). Since microorganisms can be shielded from ultraviolet light in small cracks and other shaded areas, however, these lamps are used only as a supplement to other sterilization techniques.
UV radiation can be an effective viricide and bactericide. Disinfection using UV radiation was more commonly used in wastewater treatment applications but is finding increased usage in drinking water treatment. A process named SODIS [1] has been extensively researched in Switzerland and proven ideal to treat small quantities of water. Contaminated water is filled into transparent plastic bottles and exposed to full sunlight for six hours. The sunlight is treating the contaminated water through two synergetic mechanisms: Radiation in the spectrum of UV-A (wavelength 320-400nm) and increased water temperature. If the water temperature rises above 50°C, the disinfection process is three times faster. It used to be thought that UV disinfection was more effective for bacteria and viruses, which have more exposed genetic material, than for larger pathogens which have outer coatings or that form cyst states (e.g., Giardia) that shield their DNA from the UV light. However, it was recently discovered that ultraviolet radiation can be somewhat effective for treating the microorganism Cryptosporidium. The findings resulted in two US patents and the use of UV radiation as a viable method to treat drinking water. Giardia in turn has been shown to be very susceptible to UV-C when the tests were based on infectivity rather than excystation[11]. It turns out that protists are able to survive high UV-C doses but are sterilized at low doses.
As consumer demand for fresh and "fresh like" food products increases, the demand for nonthermal methods of food processing is likewise on the rise. In addition, public awareness regarding the dangers of food poisoning is also raising demand for improved food processing methods. Ultraviolet radiation is used in several food processes to remove unwanted microorganisms. UV light can be used to pasteurize fruit juices by flowing the juice over a high intensity ultraviolet light source. The effectiveness of such a process depends on the UV absorbance of the juice (see Beer's law).
Ultraviolet detectors generally use either a solid-state device, such as one based on silicon carbide or aluminum nitride, or a gas-filled tube as the sensing element. UV detectors which are sensitive to UV light in any part of the spectrum respond to irradiation by sunlight and artificial light. A burning hydrogen flame, for instance, radiates strongly in the 185 to 260 nanometer range and only very weakly in the IR region, while a coal fire emits very weakly in the UV band yet very strongly at IR wavelengths; thus a fire detector which operates using both UV and IR detectors is more reliable than one with a UV detector alone. Virtually all fires emit some radiation in the UVB band, while the Sun's radiation at this band is absorbed by the Earth's atmosphere. The result is that the UV detector is "solar blind", meaning it will not cause an alarm in response to radiation from the Sun, so it can easily be used both indoors and outdoors.
UV detectors are sensitive to most fires, including hydrocarbons, metals, sulfur, hydrogen, hydrazine, and ammonia. Arc welding, electrical arcs, lightning, X-rays used in nondestructive metal testing equipment (though this is highly unlikely), and radioactive materials can produce levels that will activate a UV detection system. The presence of UV-absorbing gases and vapors will attenuate the UV radiation from a fire, adversely affecting the ability of the detector to detect flames. Likewise, the presence of an oil mist in the air or an oil film on the detector window will have the same effect.
Certain inks, coatings and adhesives are formulated with photoinitiators and resins. When exposed to the correct energy and irradiance in the required band of UV light, polymerization occurs, and so the adhesives harden or cure. Usually, this reaction is very quick, a matter of a few seconds. Applications include glass and plastic bonding, optical fiber coatings, the coating of flooring, paper finishes in offset printing, and dental fillings.
An industry has developed around the manufacture of UV sources for UV curing applications. Fast processes such as flexo or offset printing require high intensity light focused via reflectors onto a moving substrate and medium and high pressure Hg or Fe based bulbs are used which can be energized with electric arc or microwaves. Lower power fluorescent lamps can be used for static applications and in some cases, small high pressure lamps can have light focused and transmitted to the work area via liquid filled or fiber optic light guides. Radtech is a trade association dedicated to the promotion of this technology.
UV lights have been installed in some parts of the world in public restrooms, and on public transport, for the purpose of deterring substance abuse. The blue color of these lights, combined with the fluorescence of the skin, make it harder for intravenous drug users to find a vein. The efficacy of these lights for that purpose has been questioned, with some suggesting that drug users simply find a vein outside the public restroom and mark the spot with a marker for accessibility when inside the restroom. There is currently no published evidence supporting the idea of a deterrent effect.
Some EPROM (electronically programmable read-only memory) modules are erased by exposure to UV radiation. These modules often have a transparent glass (quartz) window on the top of the chip that allows the UV radiation in. These have been largely superseded by EEPROM and flash memory chips in most devices.
UV radiation is useful in preparing low surface energy polymers for adhesives. Polymers exposed to UV light will oxidize thus raising the surface energy of the polymer. Once the surface energy of the polymer has been raised, the bond between the adhesive and the polymer will be greater.
Using multi-spectral imaging it is possible to read illegible papyruses, such as the burned papyruses of the Villa of the Papyri or of Oxyrhynchus. The technique involves taking pictures of the illegible papyruses using different filters in the infrared or ultraviolet range, finely tuned to capture certain wavelengths of light. Thus, the optimum spectral portion can be found for distinguishing ink from paper on the papyrus surface.